36+ Alkane To Alkene
Alkane To Alkene. Alkanes are saturated with hydrogens, while alkenes are two hydrogen less than alkanes. To make them react further we need to introduce some functional group and the best way is to add halogen.

Orientation of addition if hcl adds to an unsymmetrical alkene like propene, there are two possible ways it could. C x n h 2 n + 2. The e2 elimination reaction of alkyl halide is one of the most useful method for synthesizing alkene.
aspirateur rowenta air force 18v avis ampoule led voiture avis aluminium l angle price in sri lanka aspirateur rowenta sans fil 25v
Alkane Chemie Teil 1 YouTube
The e2 elimination reaction of alkyl halide is one of the most useful method for synthesizing alkene. 10.1 synthesis of alkenes 10.1.1 dehydrohalogenation of alkyl halide. Alkanes are solid, liquid or gas at room temperature depending on the size of their molecules.to learn detailed structures, formulas, and physical properties of alkanes with faqs and videos, visit byju’s for more information. The bond is broken and two new bonds are formed.

This gives them a general formula : They react rapidly with bromine, for example, to add a br 2 molecule across the c=c double bond. Orientation of addition if hcl adds to an unsymmetrical alkene like propene, there are two possible ways it could. Alkanes are saturated with hydrogens, while alkenes are two hydrogen less than alkanes. Figure 10.1a e2.

Alkanes are solid, liquid or gas at room temperature depending on the size of their molecules.to learn detailed structures, formulas, and physical properties of alkanes with faqs and videos, visit byju’s for more information. Methane gas is the first member of the homologous series of alkanes. The bond is broken and two new bonds are formed. 10.1 synthesis of alkenes.

Alkanes are simplest organic compounds that consist of single bonded carbon and hydrogen atoms with the general formula cnh2n+2. Learn vocabulary, terms, and more with flashcards, games, and other study tools. With stronger oxidizing agent being applied, the c=c double bond of alkenes can be oxidatively cleaved, and the alkene molecule is cleaved to smaller molecules. Alkene + h 2.

The main difference between alkanes and alkenes is that alkanes are saturated hydrocarbons whereas alkenes are. An alkene represents an unsaturated hydrocarbon with double bonds, while an alkane is a saturated hydrocarbon with only single bonds. Chemical tests for alkanes, alkenes, and aromatic compounds, page 2 positive test for the presence of a double bond. Difference whatsoever between these alkenes.

Learn vocabulary, terms, and more with flashcards, games, and other study tools. This means that they have similar chemical properties to each other and they have trends in physical properties. The general formula of alkenes are c n h 2n in comparison to alkanes with general formula c n h 2n+2. Alkanes are saturated with hydrogens, while alkenes are two.
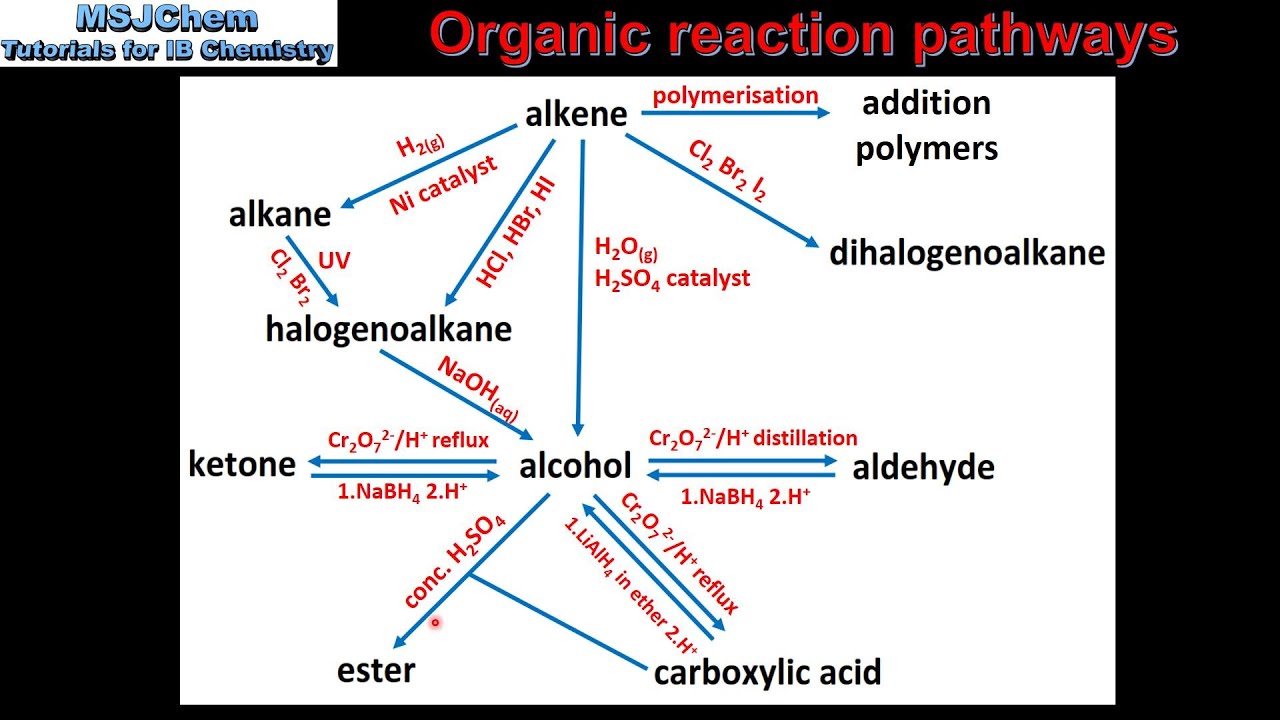
An alkane has single bonds only. Many of these molecules are used in the production of other materials, such as plastics, but. Compound composed of only carbon and hydrogen saturated hydrocarbons : Figure 10.1a e2 elimination of alkyl halide to synthesize alkene. They react rapidly with bromine, for example, to add a br 2 molecule across the c=c double bond.